Boosting Alcohol Production during Fischer-Tropsch Synthesis by Alloying Iron Catalysts with Cobalt and Nickel
on oxygenate selectivity in Fischer-Tropsch synthesis on iron alloy catalysts in ACS Catalysis
Fischer-Tropsch synthesis is the conversion of synthesis gas, CO+H2, into liquid hydrocarbon fuels, and is eminently suited for producing clean synthetic fuels by using renewable electricity for electrolysis of CO2 and water to generate syngas. In this way, green energy can effectively be stored in liquids of high energy density, which also allow utilization of the existing infrastructure for the fuels we use nowadays. Besides the possibility to make clean fuels, there is also a need for producing chemicals, for example alcohols such as ethanol, propanol, and aldehydes, which are important base chemicals for the industry. In standard Fischer-Tropsch synthesis with iron catalysts, roughly 8-10% of the converted CO molecules end up as oxygenated hydrocarbons, of which about 60-65% are alcohols.
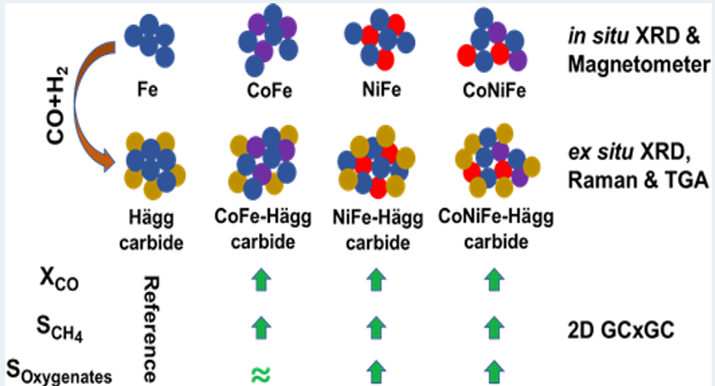
In a study sponsored by Syngaschem BV and performed mostly by Postdoc Dr Mohamed Fadlalla under supervision of Dr Nico Fischer and Prof Michael Claeys, all at the Catalysis Institute and the c*change Centre of Excellence in Catalysis at the University of Cape Town, the aim was to explore how modifying iron catalysts by alloying them with cobalt and nickel affects the selectivity towards these highly desired oxygenated products under Fischer-Tropsch conditions.It appears that alloying iron with cobalt or nickel is beneficial for the activity, as both FeCo, FeNi, and FeCoNi display higher conversion of CO. Moreover, adding Ni to iron, or both Co and Ni, leads to a doubling of the selectivities to alcohols, while the selectivity to the other oxygenates stay roughly the same. In combination with their higher activity per amount of metal in the catalyst, the yield of oxygenated products is upto a factor of 2.5 times higher than on the standard iron catalyst. The cartoon, used as the Table of Contents Graph for the article, nicely summarizes the results:
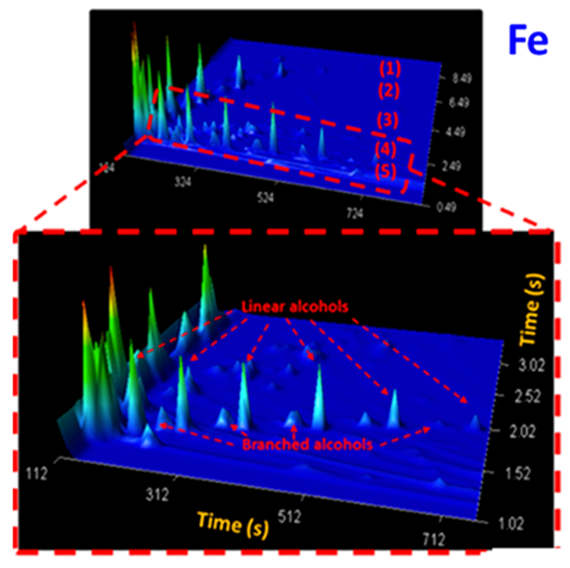
In addition to interesting observations on catalytic performance, the study addresses the physicochemical state of the catalysts with state-of-the-art characterization methods such as in situ X-ray diffraction and magnetization measurements, transmission electron microscopy, and Raman spectroscopy, while the reaction product analysis is based on 2-dimensional gas chromatography, revealing product distributions in unprecedented detail (see below).
The UCT – Syngaschem collaboration will continue with a detailed investigation of product distributions using 2D gas chromatography in a number of Fischer-Tropsch catalysts, while another study will focus on the state of the iron FT catalyst under FT conditions, using the famous in situ capabilities of the UCT laboratory.
Published on November 27, 2020