New Insights in Electro Catalysis: Magnetic Catalysts Facilitate Oxygen Evolution
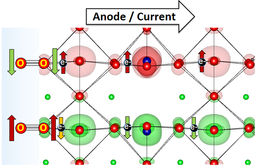
In simple terms, O2 is a molecule, in which the spins of the outer electrons orient in a parallel way, making the molecule magnetic. This is in contrast to H2 and almost all other simple molecules, which bear no net magnetic moment.
The natural way in which precursors to the O2 molecule approach each other, is with spins in opposite directions, implying that somewhere in the formation of the molecule an electron needs to flip its magnetic moment. Not only does this cost energy, there is also hardly any feasible excitation mechanism for such a transition on the surface of a catalyst.
A potentially better route towards triplet oxygen would be to ensure that the precursors (O-atoms, or an O-atom and an OH group) already meet with the spins in the right direction. This would incur a magnetic surface site which orients the adsorbates while they dock just before reaction.
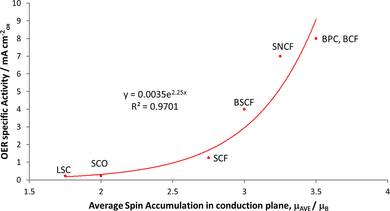
Indeed, Jose Gracia and Tingbin Lim found a remarkable correlation between the magnetic moment of antiferromagnetically ordered perovskites and their electrocatalytic reactivity for the oxygen evolution reaction, see Figure 2. Here abbreviations such as LCF or SNCF stand for perovskites such as LaCoO3 or SrNiCoFeO3-d. Antiferromagnets containing lattice planes which conduct electrons of alike spin give the best results, which to a large extent can be understood from scrutinizing their electron density of states diagrams.
This work opens the way for computational screening of electrocatalysts on the basis of the correlation between magnetic moment (a descriptor for activity), density of states and OER activity. The next challenge is of experimental and synthetic nature, as it is not always straightforward to synthesize the materials in the predicted stoichiometry.
The corresponding scientific article has been published by ChemCatChem, and is accessible via its digital object identifyer:
“Layered Antiferromagnetic Ordering in the Most Active Perovskite Catalysts for the Oxygen Evolution Reaction”
Tingbin Lim, Hans Niemantsverdriet, Jose Gracia, ChemCatChem, 23 AUG 2016
Published on August 26, 2016