Metal-Free Frustrated Lewis Pairs Successfully Capture, Release and Convert CO2
Lizette Erasmus and team publish success story in the ACS Journal Inorganic Chemistry
Carbon Dioxide Capture by Metal-Free Organometallic Complexes with a Frustration: Silica-Supported Frustrated Lewis Pairs
Carbon Capture and Storage (CCS) requires materials that not only bind CO2 – be it from exhaust streams or the air around us – but also easily release it, such that the CO2 can be stored in concentrated form. An alternative to merely storing the CO2 is to convert it into industrially important chemicals such as formaldehyde, formic acid, or methanol. This can be achieved by the reduction of CO2 with H2. As CO2 is relatively unreactive, metal catalysts and relatively harsh conditions are normally required to achieve this.
Organometallic catalysts offer avenues for activating CO2 under milder conditions, and the so-called Frustrated Lewis Pairs (FLPs) can even do this without the need for metallic components. So, what are these mysterious Frustrated Lewis Pairs? Let’s refresh our organic chemistry a bit.
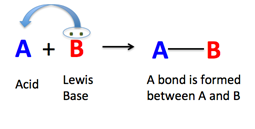
A Lewis Acid is an electron acceptor, while a Lewis Base is an electron donor, and hence if one reacts them, a stable compound forms, without much opportunity to activate other molecules. Schematically:
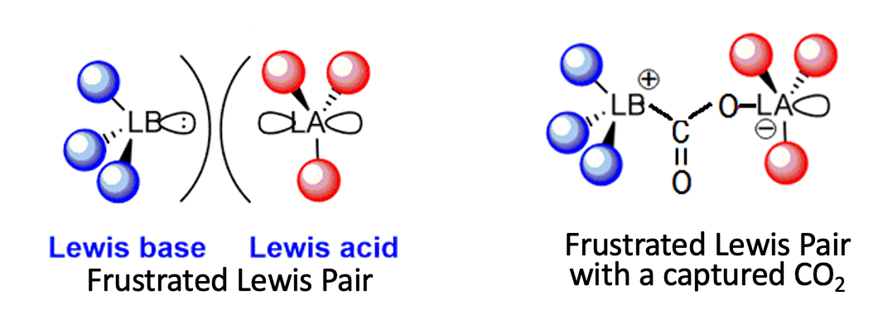
The archetype Lewis Acid is the proton, H+, and OH– is the simplest Lewis Base, which happily donates a pair of electrons to form the H-OH bond. The product is the stable water molecule, which is largely unreactive. However, if the Lewis Acid and the Lewis Base are large complexes, steric hindrance comes into play, and prevents that the acid and base centers reach each other to form a stable bond. In such cases when they still attract each other, they form a Frustrated Lewis Pair (FLP), which is much less stable than the A-B or H2O molecule above, though it may still have residual bonding power. Such complexes are generally greedy for small molecules that can help them to satisfy their hunger for chemical bonds, and CO2 is a willing victim, as the picture below illustrates. Molecules such as H2, SO2, N2O, would also be welcomed by these Frustrated Pairs.
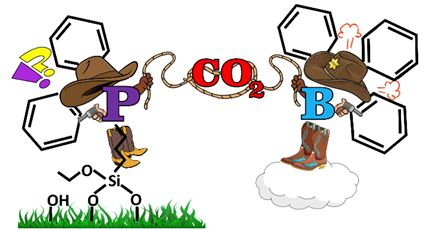
Lisette Erasmus and her students used boron-centered Lewis acids and phosporous-based Lewis bases, both with bulky ligands. Note that these systems contain no metals. For practical applications, one prefers that the active complex, the FLP, is bound to a catalyst support such as silica (SiO2), so that the eventual complex with the captured CO2 is also bound. This offers the advantage that the catalyst can eventually be used in a continuous flow reactor on an industrial scale, because the complex with captured CO2 stays in the reactor, where CO2 can be retrieved, which at the same time recycles the catalyst.
In this elegant study, the Erasmus team demonstrates that anchoring of properly functionalized Lewis acids and bases onto silica supports is feasible. The interaction between the grounded Lewis acid and a dissolved Lewis base as partner in frustration indeed produces associated heterogeneously supported Frustrated Lewis Pairs. The approach also works with the base grounded and the acid in solution. Moreover, the team demonstrated convincingly that these novel systems successfully capture CO2 at low temperature (-65 °C) and release it again at room temperature so that they are interesting candidates for carbon capture and storage (CCS).
The story was well received by the journal Inorganic Chemistry who published it on the web just before the Christmas Holidays started. We congratulate Dr Lisette Erasmus with this nice project and wish her many more successes in the future.
Cite this article
K. Mentoor, L. Twigge, J.W. Niemantsverdriet, J.C. Swarts, E. Erasmus*, “Silica Nanopowder-Supported Frustrated Lewis Pairs for CO2 Capture and Conversion to Formic Acid”, Inorganic Chemistry 60 (2021) 55-69 (published December 22, 2020)
Published on January 6, 2021